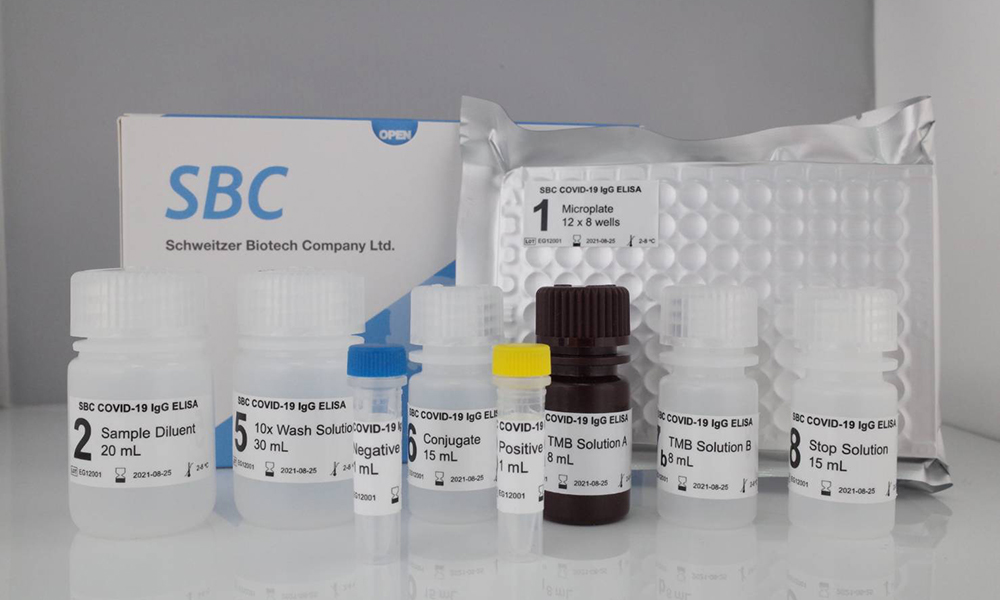
The SBC COVID-19 IgG ELISA Kit is an Enzyme-Linked Immunosorbent Assay (ELISA) intended for qualitative detection of IgG antibodies to SARS-CoV-2 in human serum.
High sensitivity and specificity
97.1% positive percent agreement (95% CI, 85.5%-99.5%)
Confirmed by testing 35 serum samples from patients that were confirmed positive for SARS-CoV-2 by US FDA authorized PCR 15- 40 days prior to serum collection.
100% negative percent agreement (95% CI, 95.8%-100%)
Confirmed by testing 87 serum samples collected in the US prior to December 2019.
Full-length spike protein coated microplates (S1 and S2)
TFDA EUA approval No.1111607835


