Introduction to COVID-19
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2).
This novel coronavirus was first identified in December 2019. In March 2020, the World Health Organization (WHO) declared the COVID-19 outbreak a pandemic.
The time between exposure to the virus and symptom onset is typically around five days but may range from two to fourteen days.
Common symptoms are similar to seasonal flu. While the majority of cases result in mild symptoms, some progress to viral pneumonia and multi-organ failure.
There is no cure available. Even with a vaccine, testing remains crucial in the fight against COVID-19.


COVID-19 testing options
Two kinds of tests are available for COVID-19: serological test and molecular test.
The serological test
The serological test detects antibodies against the virus in serum samples. The presence of antibodies indicates exposure to the virus and the body’s response to infections. Although antibodies that result from SARS-CoV-2 infection may provide some immunity, the level, and duration of immunity are not yet known.
The molecular test
The molecular test detects genetic material of the virus in respiratory specimens (such as nasopharyngeal or oropharyngeal swabs). Quantitative reverse transcription PCR (RT-qPCR) is the gold standard to diagnose COVID-19.
Serology Testing
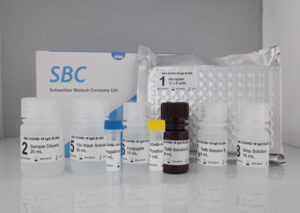
SBC COVID-19 IgG ELISA Kit
Qualitative assessment of IgG
The SBC COVID-19 IgG ELISA Kit is an Enzyme-Linked Immunosorbent Assay (ELISA) intended for qualitative detection of IgG antibodies to SARS-CoV-2 in human serum.
* High sensitivity and specificity
97.1% positive percent agreement (95% CI, 85.5%-99.5%)
Estimated by testing 35 serum samples from patients that were confirmed positive for SARS-CoV-2 by US FDA authorized PCR 15-40 days prior to serum collection.
100% negative percent agreement (95% CI, 95.8%-100%)
Estimated by testing 87 serum samples collected in the US prior to December 2019.
* Full-length spike protein coated microplates (S1 and S2).
* Microplate in strip format (adjustable to sample size)
* Compatible with various open platform ELISA analyzers
* For Research use only
Molecular Testing
- Spin column
- One-step (under development)
- Automated magnetic bead-based
- Internal control included
- High sensitivity & specificity
- Lab-based testing (Mini qPCR)
SBC COVID-19
NAT SOLUTION
Ready to use
reagents for qPCR
Design
Gene targets: ORF1ab and N
RNase P as internal control to prevent false negatives
Performance characteristics
100% match to sequences of SARS-CoV-2 isolates.
No cross reactivity with common respiratory flora and other viral pathogens.
The LoD was determined to be 10 copies/reaction (0.4 copies/µl) for both ORF1ab and N gene.
Advantage
1.5h-2hrs to obtain result (after extraction)
Our Products
SBC SARS-
CoV-2 MDx
qPCR Kit
- LoDs of ORF1ab and N gene were confirmed to be…
- Able to detect all circulating variants of SARS-CoV-2…
SBC
Covid-19
IgG-Elisa-Kit
- Detection of SARS-CoV-2 IgG antibody in human serum…
- High performance: Sensitivity 100%, specificity 99%
SBC
Mini qPCR
Thermocycler
- Compact and easy to carry; 16 wells capacity
- Quantitative and qualitative measurements; three…
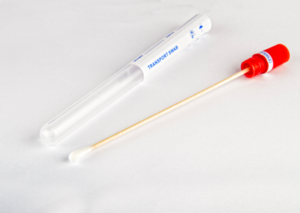
Specimen
Collection and
Transport
- Includes one oral/throat flocked tip swab and one nasopharyngeal flocked tip swab in a sterile pack…
SBC
SARS-CoV-2
qPCR Kit
- CE-IVD certification
- Validated by CLIA-certified high-complexity lab in the US using contrived samples…
SBC
RNP
qPCR Kit
- Includes a primer-probe set specific to the to the human housekeeping gene, ribonuclease P (RNP)…
Gmate®
COVID-19
Ag Saliva
- Fast results within 15 mins
- Easy to use
- Specimen: Saliva
Contact Us
Schweitzer Biotech Company Ltd.
4F, No.18, Ln.120, Sec.1, Neihu Rd., Neihu Dist.,
Taipei City 11493, TAIWAN
+886 (2) 2657 7773
If you see this Google Maps wasn't properly loaded. Please refresh your page 🙂